Eroxon® is a topical gel applied directly to the head (glans) of the penis for the treatment of male erectile dysfunction ("ED").
Mode of action
Eroxon® is a unique formulation using volatile solvent components specifically tailored for the treatment of ED. ED sufferers or their partners can apply the gel directly to the man’s penis as part of foreplay. It is fast-acting helping men get an erection within 10 minutes, and easy to use helping to restore spontaneity and intimacy in the relationship whilst offering an excellent safety profile.
Clinically proven efficacy and safety
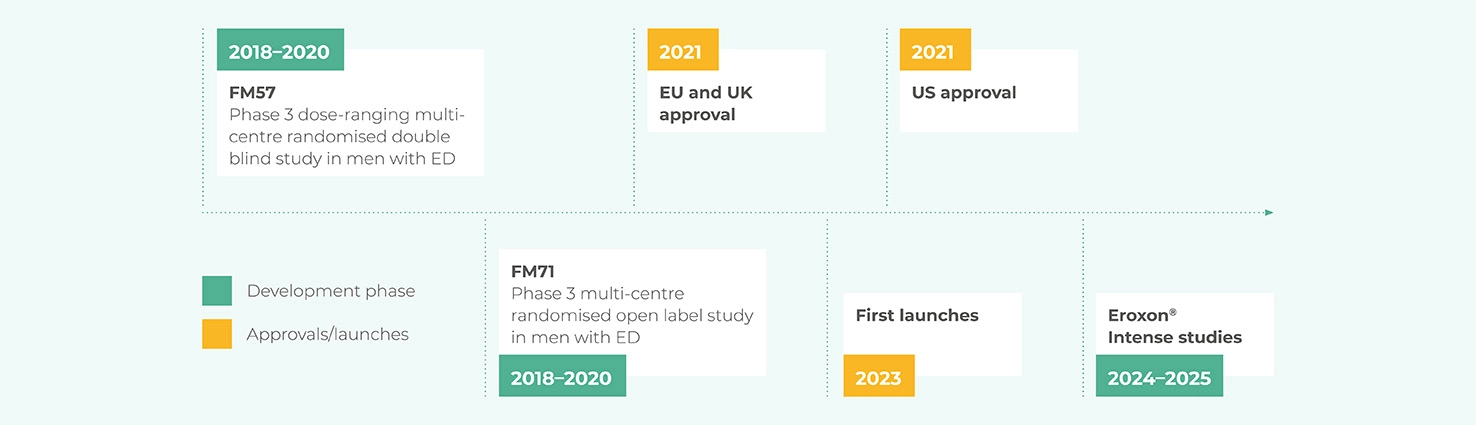
The efficacy and safety of Eroxon® was proven in two Phase 3 clinical trials conducted in Europe and the USA which were used to obtain regulatory approval in countries around the world including Europe and the USA.
60%
of erections occurred within 10 minutes of application (FM57)
63%
of men using Eroxon® met or exceeded the MCID* at 12 weeks (FM57 & FM71)
* MCID is the minimal clinically important difference (4 IIEF-EF Units) a criteria used by regulators when assessing efficacy, Rosen et al 2011
In total over 20 studies and tests were completed prior to FDA granting marketing authorisation covering safety, performance and effectiveness.
1. H1 2024 only - further launches expected in H2 2024
Excellent side effects profile
Eroxon® has an excellent side effects profile with no known drug interactions. The overall rate of side effects for the two Phase 3 studies was very low. The table below shows a list of side effects experienced by men and women that occurred in more than 1% of subjects.
| Men – Adverse events (1% or more) | Percentage of subjects |
|---|---|
| Headache | 3.0% |
| Penile burning sensation | 1.0% |
| Women – Adverse events (1% or more) | Percentage of subjects |
|---|---|
| Headache | 1.3% |
The table above lists adverse events that occurred at 1% or more in the clinical studies when the data is combined (FM57 and FM71). The adverse events are Treatment Emergent Adverse Events defined as AEs that begin after the start of trial medication and the percentages are based on the combination of the side effects for both studies with 297 subjects.
