WSD4000 is a topical treatment designed for symptoms of impaired sexual response and function in women. Currently, no regulatory approved topical treatment for symptoms of impaired sexual response and function in women is available over the counter.
WSD4000 has the potential to be an effective, breakthrough treatment for the common symptoms associated with sexual dysfunction, such as lack of arousal, lubrication and desire.
Significant unmet needs in women’s sexual health
We commissioned Ipsos to carry out independent market research in the USA in 2024 to understand the unmet needs in women with symptoms of impaired sexual response and function as well as how common these symptoms were and what women thought of our product concept for WSD4000. The research included interviews with 40 women and an online survey with 1,000 women aged 22 to 75.
The results showed that symptoms are very common, affecting 6 in 10 women over the last twelve months. What we also saw is that this is not an issue that affects older women more than younger women. The percentage of women experiencing at least one symptom was similar across the age groups, with variations in the symptoms experienced. Most women experience more than one symptom and often there are causal relationships between the various symptoms. Many women are dissatisfied with the amount of sex they are having and wish they were having more.
INCIDENCE OF SYMPTOMS OF IMPAIRED SEXUAL RESPONSE AND FUNCTION IN WOMEN*
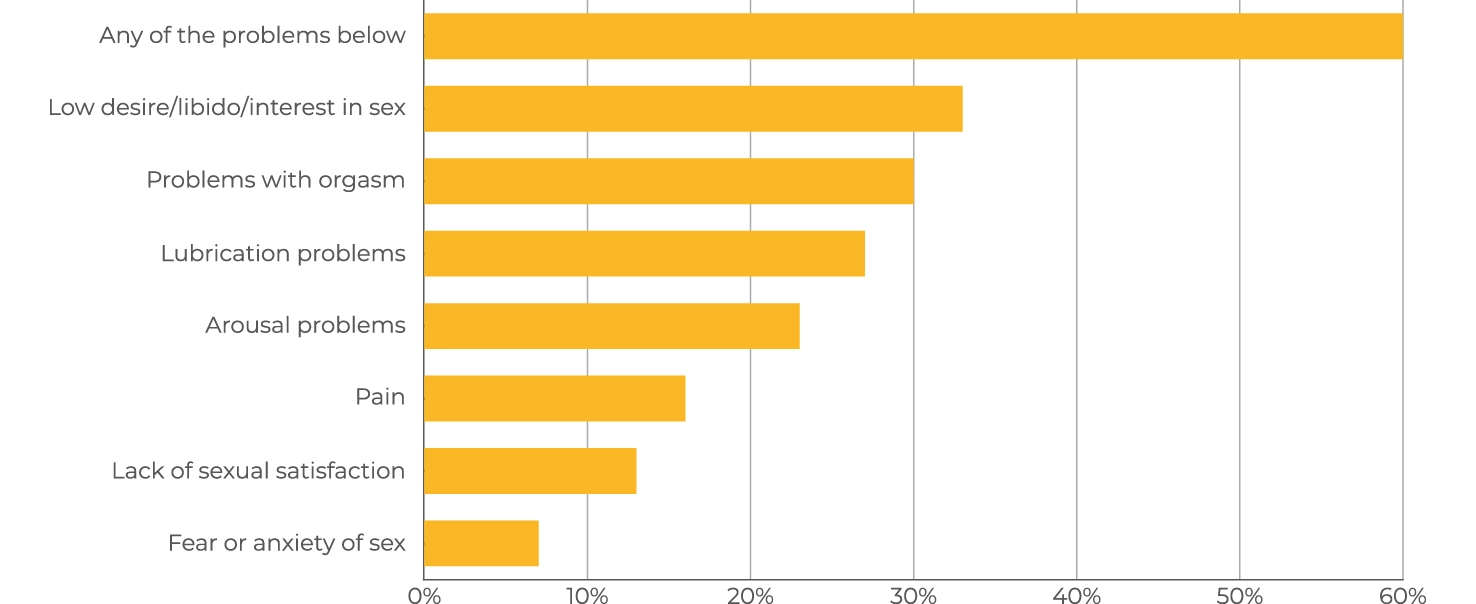
* This list consists of symptoms of impaired sexual response and function in women that WSD4000 is expected to treat and that women had experienced over the last twelve months.
Promising early home user study results
Futura conducted a sensory study at the end of 2024 which comprised 67 women, which included women suffering from some degree of symptoms of impaired sexual response and function, which delivered an overall positive change in sexual function after four weeks. The majority of respondents reported increased vaginal lubrication, increased genital sensation, improved genital pleasure and an improvement in their satisfaction with the sexual experience. 57% of women used the product on more occasions than the stated minimum which is a strong indication of the respondents' positive response to the product. 87% of women reported they would like to continue using the product. In those that experienced some degree of symptoms of impaired sexual response and function, there was a notable uplift from the baseline with positive responses in arousal, lubrication, orgasm, satisfaction and discomfort (pain).
Regulatory strategy
A further pre-submission meeting with the US FDA has taken place, where good progress was made to clearly define the product’s indications for use, the potential claims, and how these should be proven during the clinical phase of development. Following the FDA meeting and the success of the study we are conducting an Early Feasibility Study to be completed in the first half of 2026. This will enable the Group to consider refinements to the methodology in a population more representative of the target user and therefore hope to increase efficacy still further, as well as further inform on perceptions of the product. WSD4000 has the potential to be an effective breakthrough treatment for the common symptoms of symptoms of impaired sexual response and function in women and is a large and significant commercial opportunity in a market where there are very few treatments available to women.
Market opportunity
The reaction from women to the concept for WSD4000 was very positive. The concept was well received and resonated with women who felt it was written by women for women with 88% of respondents in the market research saying they would not change anything about the concept. As a concept it scored at the top of the Ipsos norms average with three areas scoring well above average including in particular “New and Different”. Interest was high and, depending on the price point, up to around 60% of women were likely or very likely to purchase WSD4000. The concept resonated particularly well in younger women.
The commercial opportunity is large and significant with an estimated 34 million women in the USA alone motivated to treat their symptoms of sexual dysfunction and 2 in 3 women dissatisfied with their treatment.
New Intellectual property
A new patent for WSD4000 was filed in February 2024, with the potential for further filings based on the outcomes of the studies. The innovation behind WSD4000 falls outside of existing commercial partner agreements for Eroxon®.
- Source: McCabe MP, Sharlip ID, Lewis R, Atalla E, Balon R etal. Incidence and Prevalence of Sexual Dysfunction in Women and Men: A Consensus Statement from the Fourth International Consultation on Sexual Medicine 2015. J Sex Med. 2016 Feb;13(2):144-52
- OTC - over the counter, not requiring a doctor's prescription
